We combine structural biology, biochemical, and biophysical approaches to study the structure and mechanism of transmembrane transport proteins. We aim to reveal the molecular basis for their physiological and pathophysiological functions.

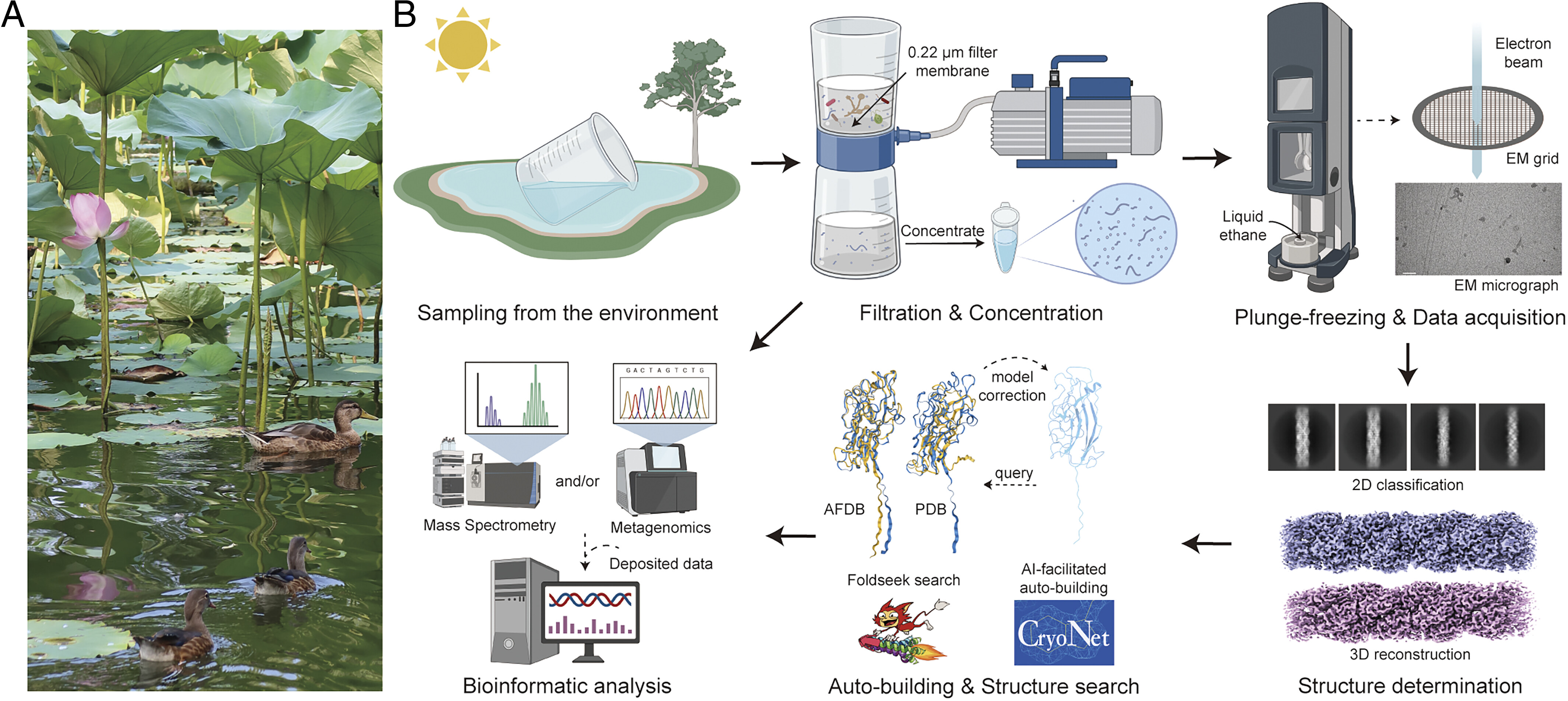
Structural biology is experiencing a paradigm shift from targeted structural determination to structure-guided discovery of previously uncharacterized bioentities.
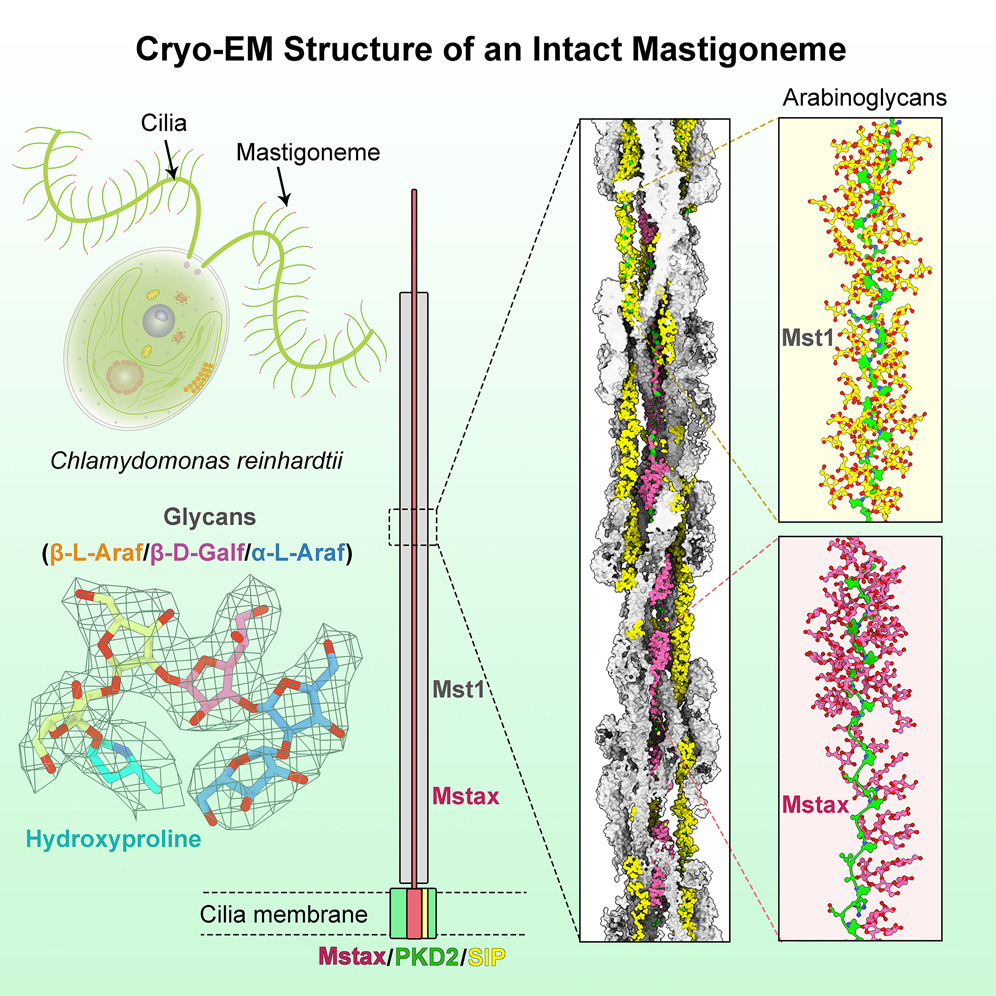
Mastigonemes, the hair-like lateral appendages lining cilia or flagella, participate in mechanosensation and cellular motion, but their constituents and structure have remained unclear. Here, we report the cryo-EM structure of native mastigonemes isolated from Chlamydomonas at 3.0 Å resolution. The long stem assembles as a super spiral, with each helical turn comprising four pairs of anti-parallel mastigoneme-like protein 1 (Mst1). A large array of arabinoglycans, which represents a common class of glycosylation in plants and algae, is resolved surrounding the type II poly-hydroxyproline (Hyp) helix in Mst1. The EM map unveils a mastigoneme axial protein (Mstax) that is rich in heavily glycosylated Hyp and contains a PKD2-like transmembrane domain (TMD). Mstax, with nearly 8,000 residues spanning from the intracellular region to the distal end of the mastigoneme, provides the framework for Mst1 assembly. Our study provides insights into the complexity of protein and glycan interactions in native bio-architectures.
Structural biology is experiencing a paradigm shift from targeted structural determination to structure-guided discovery of previously uncharacterized bioentities. We employed cryoelectron microscopy (cryo-EM) to analyze filtered water samples collected from the Tsinghua Lotus Pond. Here, we report the structural determination and characterization of two highly similar helical fibrils, named TLP-1a and TLP-1b, each approximately 8 nm in diameter with a 15-Å wide tunnel. These fibrils are assembled from a similar protein protomer, whose structure was conveniently automodeled in CryoNet. The protomer structure does not match any available experimental structures, but shares the same fold as many predicted structures of unknown functions. The amino-terminal β strand of protomer n + 4 inserts into a cleft in protomer n to complete an immunoglobulin (Ig)-like domain. This packing mechanism, known as donor-strand exchange (DSE), has been observed in several bacterial pilus assemblies, wherein the donor is protomer n + 1. Despite distinct shape and thickness, this reminiscence suggests that TLP-1a/b fibrils may represent uncharacterized bacterial pili. Our study demonstrates an emerging paradigm in structural biology, where high-resolution structural determination precedes and drives the identification and characterization of completely unknown objects.
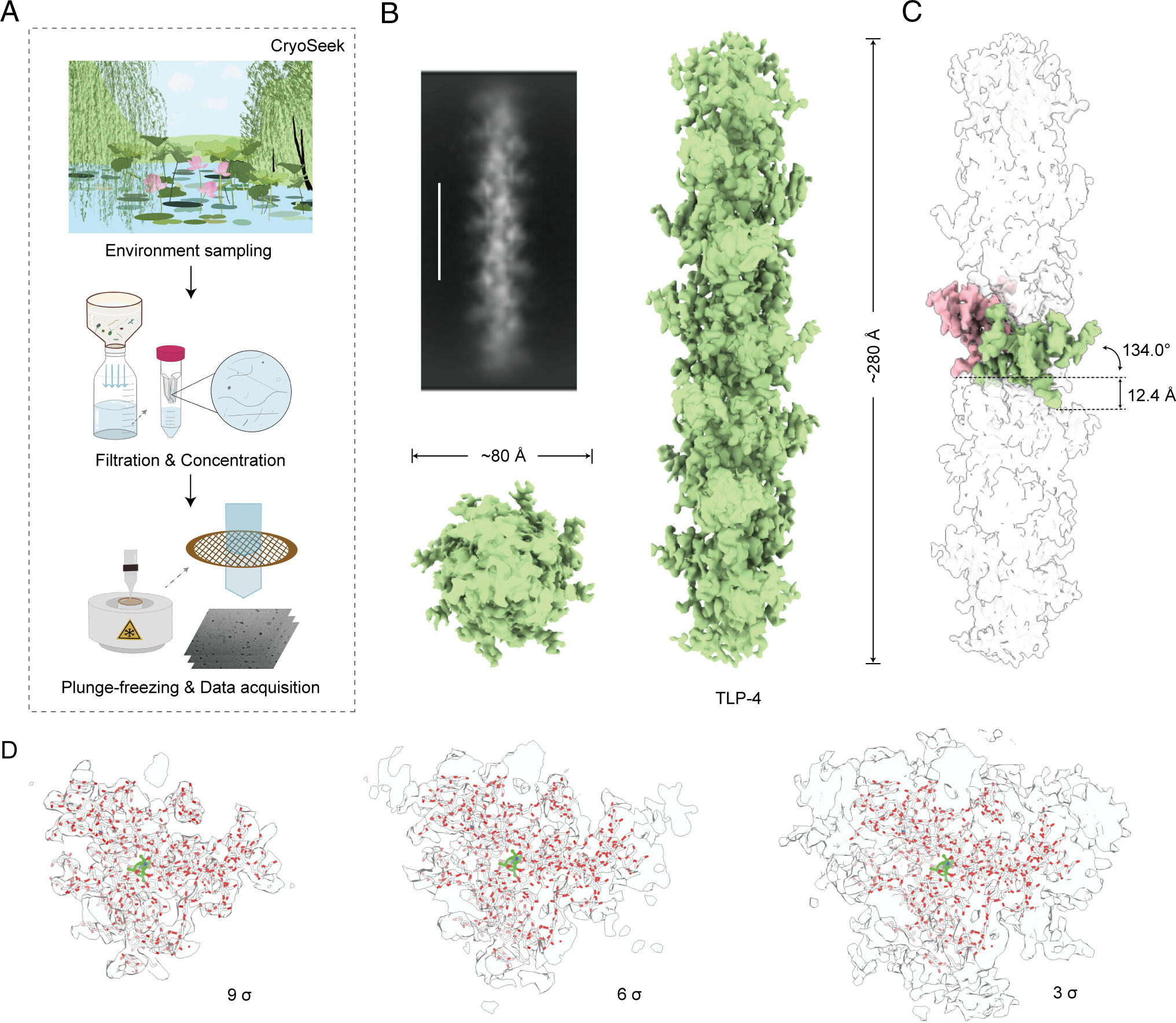
Despite the recent breakthrough in structure determination and prediction of proteins, the structural investigation of carbohydrates remains a challenge. Here, we report the cryo-EM analysis of a glycofibril found in the freshwater in the Tsinghua Lotus Pond. The fibril, which we name TLP-4, is made of a linear chain of tetrapeptide repeats coated with >4 nm thick glycans. In each repeat, two glycans are O-linked to a 3,4-dihydroxyproline and another glycan attaches to the adjacent Ser or Thr. The fibril structure is entirely maintained through glycan packing. Bioinformatic analysis confirms the conservation of the TLP-4 repeats across species, suggesting the existence of a large number of glycofibrils to be discovered. Our findings not only provide valuable insights into the structural roles of glycans in bio-assemblies but also demonstrate the potential of our recently formulated research strategy of CryoSeek to find bioentities and establish prototypes for structural studies of carbohydrates.