We combine structural biology, biochemical, and biophysical approaches to study the structure and mechanism of transmembrane transport proteins. We aim to reveal the molecular basis for their physiological and pathophysiological functions.

Cholesterol is one of the most important fundamental molecules in living organisms. In addition to helping maintain the integrity and curvature of cell membranes, cholesterol and its derivatives participate in various of physiological activities, including the synthesis of steroid hormones, signal transduction, and the assembly of lipoproteins. Over the past decade, our lab has focused on proteins and protein complexes regulated by sterols or involved in sterol metabolism. We are gradually uncovering some of the details within this vast field of study.
Cholesterol homeostasis is controlled by the SREBP (sterol regulatory element-binding protein) pathway. Key players in this pathway include SREBPs (SREBP1a, 1c and 2), Insigs (Insig1 and 2) and SCAP (SREBP cleavage activating protein). SREBPs are membrane-bound transcription factors that regulate the majority of genes related to intracellular cholesterol accumulation, and their activities are rigorously controlled by feedback mechanisms based on intracellular sterol levels. Briefly, when cells are depleted of sterols, SREBPs are translocated from the ER to Golgi apparatus under the escort of SCAP, undergoing a two-step proteolytic cleavage that releases N-terminal transcriptional domain. Conversely, when cells are replete with sterols, SCAP interacts with Insigs to retain SREBPs in the ER. The molecular details of how the SREBP-SCAP complex is regulated by Insigs and intracellular sterols have long been an enigma in this field.
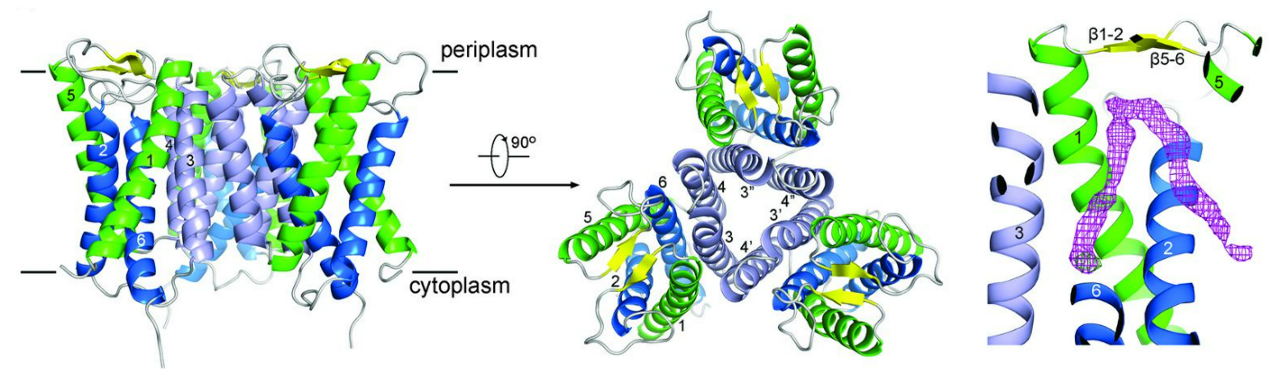
To investigate this issue, we began with homologues of SREBP and SCAP in fission yeast. These two proteins, named Sre1 and Scp1, respectively, collaboratively participate in hypoxic adaptation in fission yeast. Our crystal structures of C-terminal domain (CTD) of Sre1 and Scp1 revealed that the WD40 domain of yeast Scp1 exhibites distinctive features compared with canonical ones, and two Scp1 elements are critical for Sre1 recognition (Cell Res. 25,401-11. 2015). Sre1-CTD and Scp1-CTD form a functional 4:4 oligomer, with Sre1-CTD forming a dimer of dimers. Proper 4:4 oligomeric complex is essential for Sre1 activation in fission yeast (Cell Res. 26,1197-1211. 2016). In 2015, we also determined the crystal structure of an Insig homolog, which we named MvINS in mycobacteria. Existing as a monotrimer, the six transmembrane segments (TMs) of MvINS enclose a V-shaped cavity capable of accommodating a diacylglycerol (DAG) molecule. A homology-based structural model of human Insig2 suggested the presence of 25-hydroxycholesterol (25-HC) in this cavity (Science. 349,187-91. 2015). These foundational works laid the groundwork for our subsequent exploration and understandings of human SCAP-Insig2 complex.
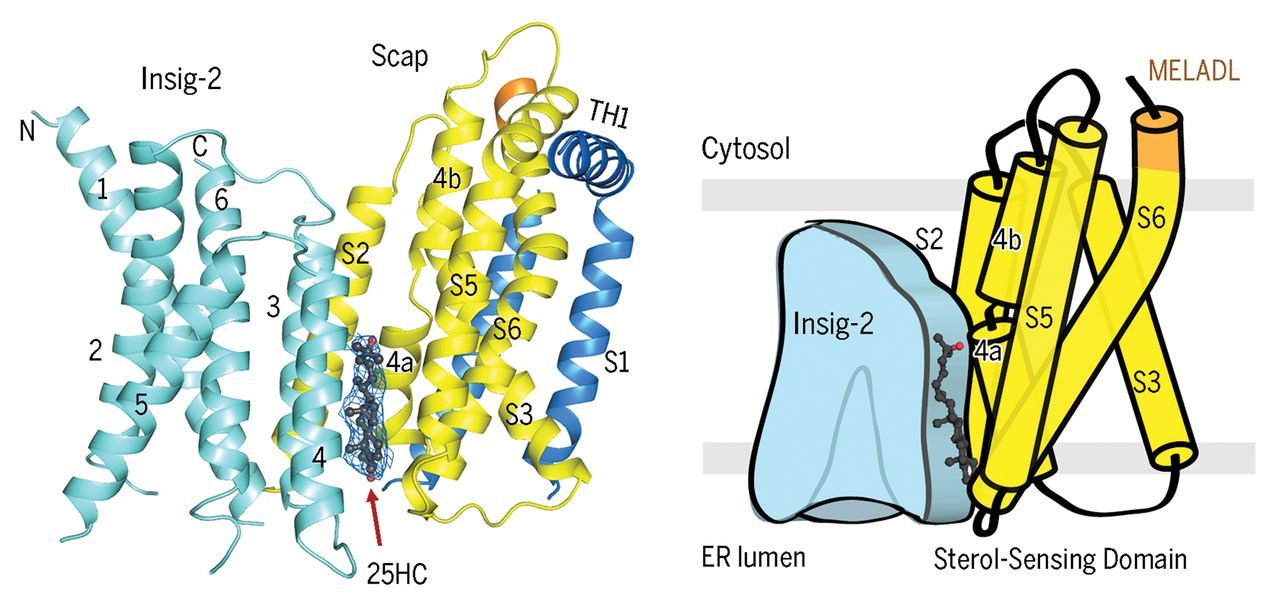
Understanding the sterol-mediated interaction between SCAP and Insigs is crucial not only for unraveling the basis of sterol sensing in this complex but also for the development of potential drugs against viral infection and cancer therapy. In our cryo-EM structures of human SCAP-Insig2 complex, we unambiguously assigned the 25-HC molecule at the interface of SCAP and Insig2, which differs from our previous hypotheses and the prevailing perceptions. The binding site is primarily constituted by hydrophobic residues on TM3 and TM4 of Insig-2 and S4, S5, and S6 of Scap. The 25-OH group at the end of the iso-octanol tail of 25-HC is exposed to the cytosolic milieu through a hydrophilic cavity enclosed by Scap and Insig-2, providing a potential explanation for the preference of 25HC over cholesterol in promoting the interaction between Scap and Insigs (Science. 371, eabb2224. 2021).
While SCAP-Insig2 complex with 25-HC reveals a promising interpretation for the 25-HC-dependent interaction between SCAP and Insigs, how intracellular cholesterol levels regulate complex formation remained intriguing, and research has suggested the existence of a different mechanism. By excluding 25-HC during complex purification, we identified four densities for digitonin (a detergent that shares a similar scaffold with cholesterol), one at the interface where 25-HC used to exist, two in the central pocket of Insig2 and one on the surface constituted by TM3/5/6 of Insig2. The two densities in the central pocket are in consistent with that observed in MvINS (Cell Rep. 35,109229. 2021).
The Hedgehog (Hh) pathway is critical for embryogenesis and regeneration. Activation of this pathway requires the binding of the secreted and lipid-modified protein Hh to the membrane receptor Patched (Ptch); otherwise, Ptch suppresses the downstream G protein-coupled receptor Smoothened (Smo). Generally, mammals have three Hh homologs: Sonic (Shh), Desert (Dhh), and Indian (Ihh), with Shh serving as the prototype for functional and mechanistic studies. The Shh precursor, with approximately 450 residues, undergoes autocatalytic cleveage to yield N-terminal ShhN, which is modified by palmitoylation at the N-terminal and cholesterol at the C-terminal for further functionality.
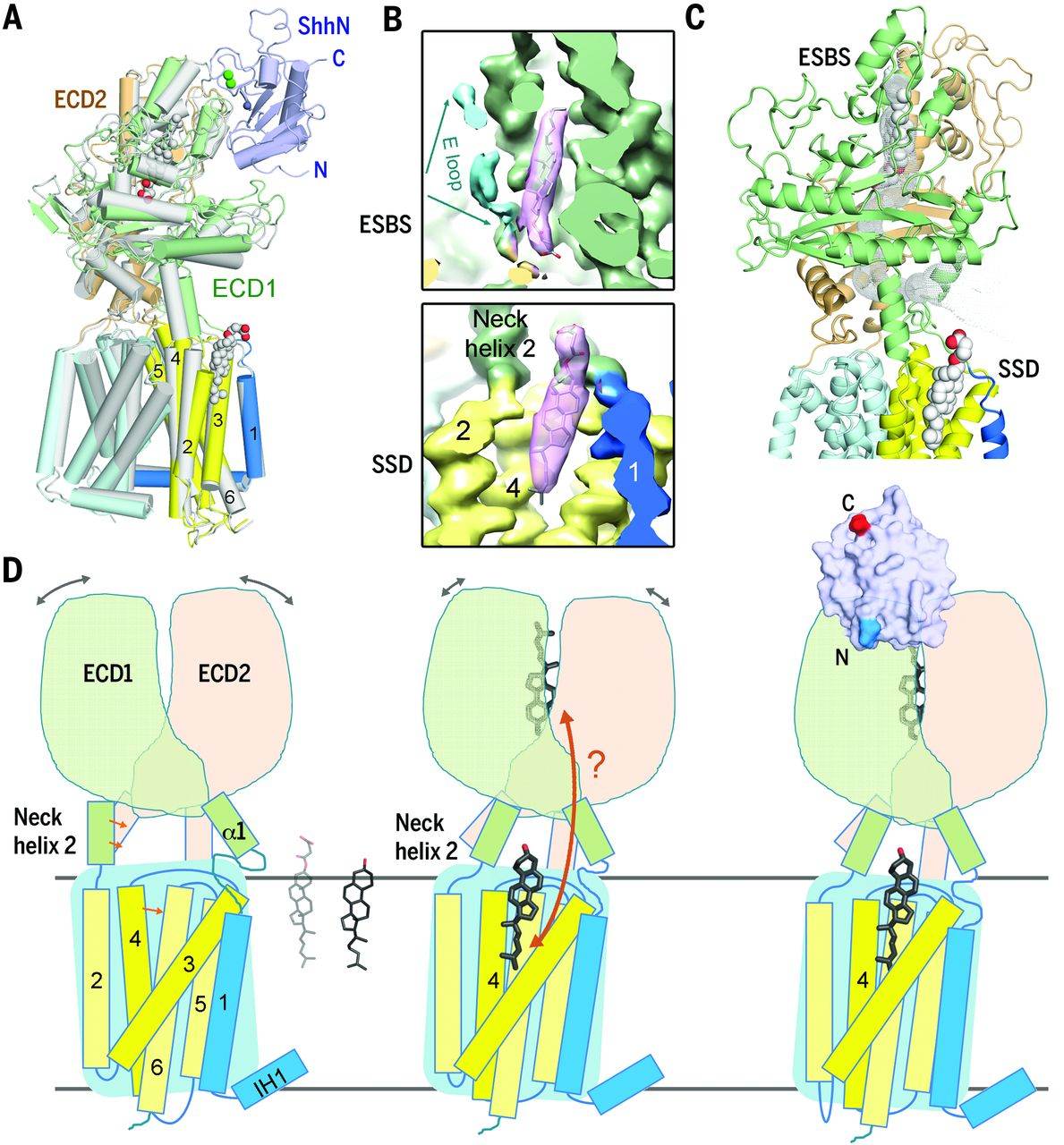
In our published cryo-EM structure of the Ptch1 and Ptch1-ShhN complex, we identified two steroid densities in both structures, one in the pocket enclosed by the extracellular domain (ECD) and the other in the membrane-facing cavity of sterol-sensing domain (SSD). Ptch1 undergoes steroid-induced conformational changes, and the binding of ShhN locks Ptch1 into a stable conformation (Science. 361, eaas8935. 2018).
Further investigation into these two proteins revealed that Ptch1 forms a complex with palmitoylated human Shh (ShhNp) at a 4:2 stoichiometric ratio. Five cholesterol-like densities were observed in the map, with four occupying previously identified sites and an extra one between SSD and ECD. The binding interfaces between one ShhNp and two Ptch1 are asymmetric, suggesting two distinct inhibitory mechanism for the two Ptch1 protomers by one ShhNp (Nat Commun. 10,2320.2019).
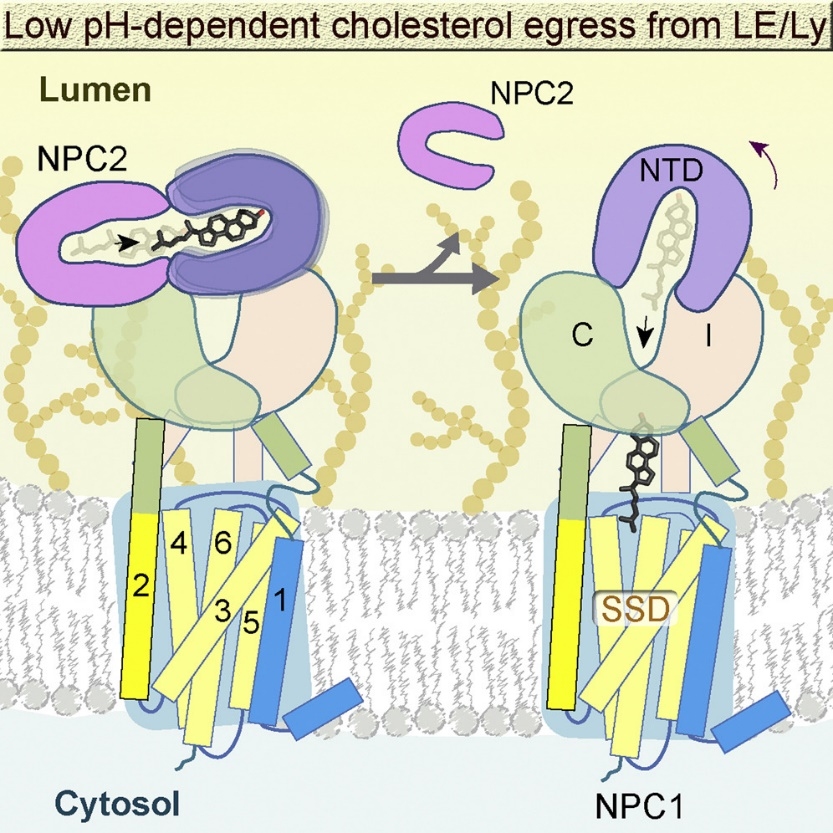
Niemann-Pick C1 (NPC1) and NPC2 cooperate to export LDL (low-density lipoprotein)-derived cholesterol from late endosomes and lysosomes to the other cellular compartments. NPC1 is a polytopic membrane protein located in the membrane of endosomes and lysosomes, while NPC2 is small soluble protein in the lumens. In 2016, we elucidated the cryo-EM structures of full-length human NPC1 and NPC1 in complex with the cleaved surface glycoprotein (GPcl) of the Ebola virus (EBOV), as NPC1 also mediates EBOV cellular entry. These structures help clarifythe mechanisms underlying NPC1-mediated intracellular cholesterol trafficking and EBOV infection (Cell. 165,1467-1478).
In 2020, we further discovered that NPC1 adopts different conformations under different pH conditions. Compared with pH 8.0, the N-terminal domain (NTD) of NPC1 at pH 5.5 exhibites increased flexibility, facilitating cholesterol transfer from the NTD to the central tunnel of NPC1. We also obtained the cryo-EM structure of the NPC1-NPC2 complex at pH 5.5. By analyzing NPC1 at different pH levels, we elucidated the molecular basis for the handoff of cholesterol from NPC2 to NPC1 (Cell. 182,98-111. 2020).
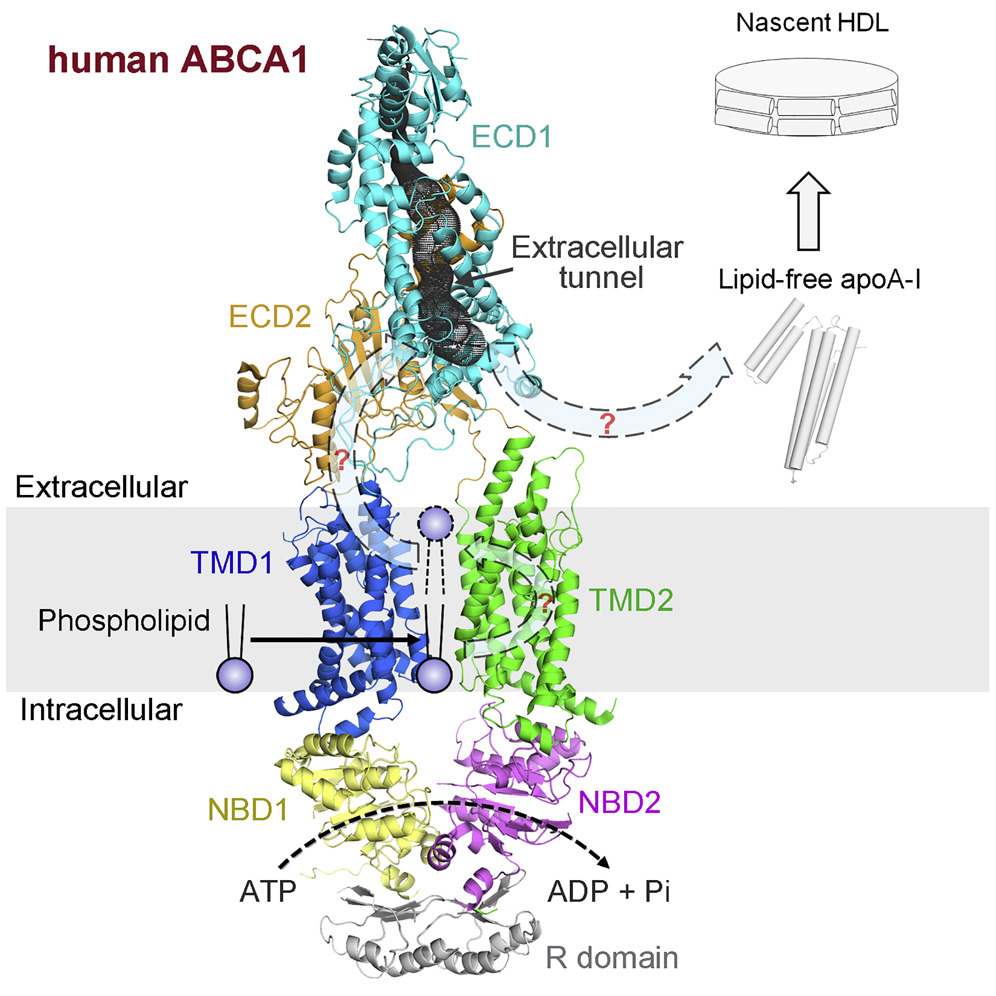
ATP-binding cassette (ABC) superfamily A exporter mediates cellular efflux of phospholipids and cholesterol to the extracellular acceptor apoA-I for the generation of nascent high-density lipoprotein (HDL). Our cryo-EM structure of ABCA1 revealed that the transmembrane domain (TMD) of ABCA1 exhibits an “outward-facing”conformation, even in the absence of nucleotide, which is distinct from any other known structure of the nucleotide-free ABC exporters. This structure also suggests that pronounced conformational changes must occur for the substrates to be delivered from the membrane to the hydrophobic tunnel within the ECD (Cell. 169,1228-1239. 2017).
The membrane-bound O-acyltransferase (MBOAR) superfamily emcompasses a diverse array of enzymes involved in the acylation of lipids and proteins.
Acyl-coenzyme A:cholesterol acyltransferases (ACATs) catalyze the transfer of an acyl group from the acyl-coenzyme A to cholesterol, generating cholesteryl ester, the primary form in which cholesterol is stored in cells and transported in plasma. Human ACAT1, according to our published structure, exists as a dimer of dimers. Each protomer of ACAT1 consists of nine TM segments, enclosing a cytosolic tunnel and a transmembrane tunnel that converge at the predicted catalytic site. Together with structure-based biochemical assays, we postulated that acyl-coenzyme A enters the active site through the cytosolic tunnel, whereas cholesterol may enter from the side through the transmembrane tunnel (Naure. 581,33-338. 2020).
Diacyglycerol O-acyltransferase 1 (DGAT1) synthesizes triacylglycerides and is essential for dietary fat absorption and fat storage in humans. The cryo-EM structure of DGAT1 in complex with an oleoyl-CoA substrate revealed a hollow chamber enclosed by highly conserved catalytic residues, with separate entrances for each of the two substrates: fatty acyl-CoA and diacylglycerol (Nature. 581, 329-332.2020).